The report analyzes the market of devices by 6 segments such as cardiac rhythm management devices, electrocardiogram, holter monitors, event monitors, implantable loop recorder, and cardiac output monitoring devices. All of these segments experienced a positive growth till 2012, owing to the increased awareness for procedures and sophisticated diagnostic techniques. Cardiac rhythm management devices segments have been contributing nearly 50%, in terms of value, towards the North American market.
In North America, sudden cardiac arrest (SCA) is the leading cause of death, especially among the U.S. adults aged 40 years and above. Moreover, the awareness about these diseases and availability of various monitoring and therapeutic devices is also increasing, further accelerating the growth of market. The primary growth driver for the North America market remains the rising incidence of cardiovascular diseases.
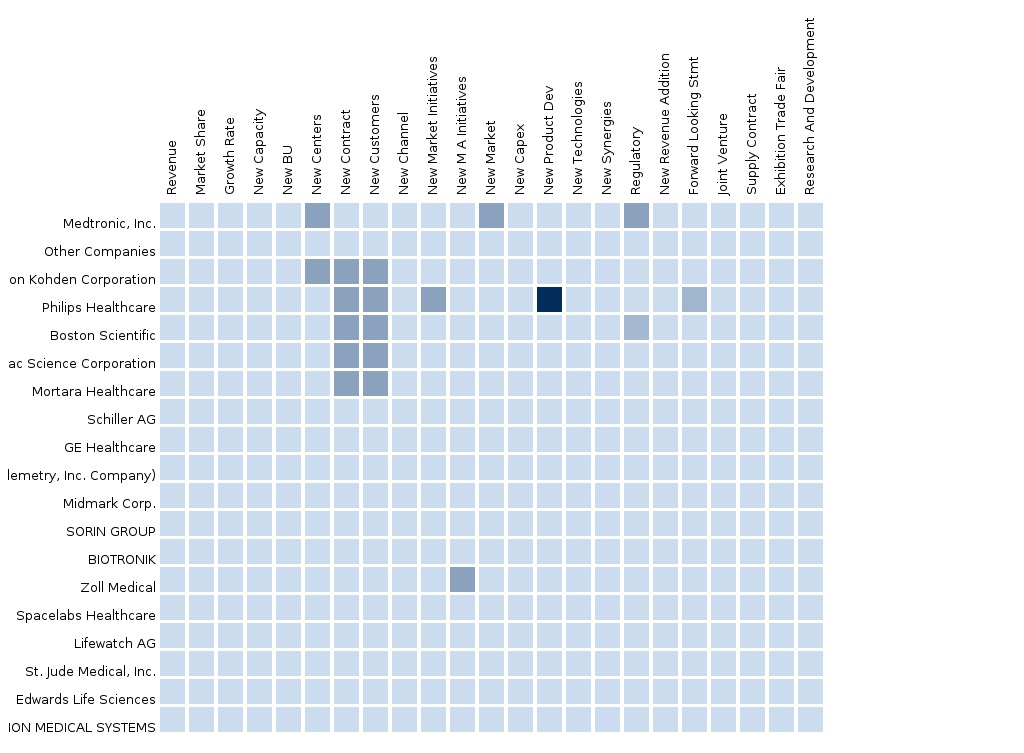
The report also provides an extensive competitive landscaping of the leading companies operating in this market. The main companies operating in the cardiac monitoring devices market and extensively covered in this report are Medtronic, St. Jude Medical, Boston Scientific, Philips Healthcare, GE Healthcare, Nihon Kohden, Cardiac Science, CardioNet, LifeWatch, Spacelabs, Mortara Instrument, Welch Allyn, and Schiller AG. The segment and country-specific company shares, news & deals, M&A, segment-specific pipeline products, product approvals, and product recalls of the major companies have been detailed.
Customization Options:
Along with market data, customize the MMM assessments to meet your company’s specific needs. Customize to get a comprehensive summary of the industry standards and a deep dive analysis of the following parameters:
Product Analysis:
- Usage pattern (in-depth trend analysis) of products (segment-wise)
- Product matrix, which gives a detailed comparison of product portfolio of each company, mapped at country and sub-segment levels
- Adoption rate analysis of the products (segment-wise and country-wise)
Epidemiology Data:
- Country-specific prevalence of CVD
- Country-specific patient pool of CVD
- Disease progression (pattern analysis)
Procedure Volume Data:
- Number of CV procedures performed in each country
- End-users
Surgeons/Physicians Perception Matrix:
- Fast turn-around analysis of surgeons’ responses to market events and trends
- Pattern analysis of usage of devices by physicians
- Surgeons’ opinions about products from different companies
- Surgeons’ qualitative inputs on epidemiology data
Brand/Product Perception Matrix:
- A comprehensive study of customers perception and behavior through our inbuilt social connect tool checking the virality and tonality of blogs
- An analysis of overall brand usage and familiarity and brand advocacy distribution (Detractor/Neutral/Familiar)
Alternative Products-Impact analysis:
- MMM’s Healthcare Decision Making Quadrant It is an innovative and useful quadrant for vendors who wish to analyze the potential growth markets based on parameters such as patient dynamics (patient pool, epidemiology of disease, preference towards surgeries/alternative therapies) and macroeconomic indicators (number of hospitals and orthopedic clinics, reimbursement scenario, diagnosis rate, treatment rate and healthcare expenditure)
1 Introduction
1.1 Objective of the study
1.2 Market Definitions
1.3 Market Segmentation & Aspects Covered
1.4 Research Methodology
1.4.1 Assumptions (Market Size, Forecast, etc)
2 Executive Summary
3 Market Overview
4 Cardiac Monitoring Devices-North America, By Segments
4.1 Split By Geography
4.1 Cardiac Monitoring Devices-USA by Markets
4.1 Cardiac Monitoring Devices-Canada by Markets
4.2 Event Monitors-North America
4.2.1 Event Monitors-North America, By Geographies
4.2.1.1 Event Monitors-USA
4.2.1.2 Event Monitors-Canada
4.2.2 Event Monitors-North America, By Services
4.2.2.1 Event Monitoring-North America
4.2.3 Event Monitors-North America, By Segments
4.2.3.1 Post-symptom-North America
4.2.3.2 Pre-symptom-North America
4.2.4 Event Monitors-North America, By Technologies
4.2.4.1 Auto-detect Event Monitor-North America
4.2.4.2 Manual Event Monitor-North America
4.3 Holter Monitors-North America
4.3.1 Holter Monitors-North America, By Geographies
4.3.1.1 Holter Monitors-USA
4.3.1.2 Holter Monitors-Canada
4.3.2 Holter Monitors-North America, By Segments
4.3.2.1 Wired Holter Monitors-North America
4.3.2.2 Wireless Holter Monitors-North America
4.4 Implantable Loop Recorder-North America
4.4.1 Implantable Loop Recorder-North America, By Geographies
4.4.1.1 Implantable Loop Recorder-USA
4.4.1.2 Implantable Loop Recorder-Canada
4.5 Cardiac Output Monitoring Devices-North America
4.5.1 Cardiac Output Monitoring Devices-North America, By Geographies
4.5.1.1 Cardiac Output Monitoring Devices-USA
4.5.1.2 Cardiac Output Monitoring Devices-Canada
4.5.2 Cardiac Output Monitoring Devices-North America, By Products
4.5.2.1 Minimally Invasive Cardiac Output Monitoring Device-North America
4.5.2.2 Non-invasive Cardiac Output Monitoring Device-North America
4.6 Electrocardiogram-North America
4.6.1 Electrocardiogram-North America, By Geographies
4.6.1.1 Electrocardiogram-USA
4.6.1.2 Electrocardiogram-Canada
4.6.2 Electrocardiogram-North America, By Segments
4.6.2.1 Resting ECG-North America
4.6.2.2 Stress ECG-North America
5 Cardiac Monitoring Devices-North America, By Geographies
5.1 Cardiac Monitoring Devices-USA
5.1.1 Cardiac Monitoring Devices-USA, By Segments
5.1.1.1 Electrocardiogram-USA
5.1.1.2 Holter Monitors-USA
5.1.1.3 Event Monitors-USA
5.1.1.4 Implantable Loop Recorder-USA
5.1.1.5 Cardiac Output Monitoring Devices-USA
5.2 Cardiac Monitoring Devices-Canada
5.2.1 Cardiac Monitoring Devices-Canada, By Segments
5.2.1.1 Electrocardiogram-Canada
5.2.1.2 Holter Monitors-Canada
5.2.1.3 Event Monitors-Canada
5.2.1.4 Implantable Loop Recorder-Canada
5.2.1.5 Cardiac Output Monitoring Devices-Canada
Please fill in the form below to receive a free copy of the Summary of this Report
Please visit http://www.micromarketmonitor.com/custom-research-services.html to specify your custom Research Requirement














