You are here :
Home
Healthcare
Payment Link for Sterlitech Corporation - Track etched Membrane Market & Pharmaceutical Filtration Market
Drug formulations
Chronic myeloid leukemia (CML)
Bosutinib and Lab-on-chips (Microfluidics), Microreactors, AMG 103, DFP-10917, Tasigna and BP-100-1.01 adds up to total Pipeline Drugs (CML) market.
Bosutinib can be segmented by Submarkets, Technologies, Products, Geographies, Companies and Phases. Submarkets of this market are Chromatography, E-Clinical Trial Solutions, Wireless Health, Clinical Trial Management Systems (CTMS), Healthcare IT outsourcing, Immunoassay, Digital Pathology, Chromatography Instruments, Next Generation Sequencing (NGS) and Proteomics. Technologies of this market are Microfluidics and Chromatography Reagents. Products of this market are Lab-on-chips (Microfluidics), Microreactors, AMG 103, DFP-10917, Tasigna and BP-100-1.01. Geographies of this market are USA, Canada, United Kingdom, Germany, France, Italy, Spain and Japan. Companies of this market are Pfizer Inc, Novartis AG, ARIAD Pharmaceuticals Inc and BRISTOL-MYERS SQUIBB. Phases of this market are Phase II.
Bosutinib can be segmented by Submarkets, Technologies, Products, Geographies, Companies and Phases. Submarkets of this market are Chromatography, E-Clinical Trial Solutions, Wireless Health, Clinical Trial Management Systems (CTMS), Healthcare IT outsourcing, Immunoassay, Digital Pathology, Chromatography Instruments, Next Generation Sequencing (NGS) and Proteomics. Technologies of this market are Microfluidics and Chromatography Reagents. Products of this market are Lab-on-chips (Microfluidics), Microreactors, AMG 103, DFP-10917, Tasigna and BP-100-1.01. Geographies of this market are USA, Canada, United Kingdom, Germany, France, Italy, Spain and Japan. Companies of this market are Pfizer Inc, Novartis AG, ARIAD Pharmaceuticals Inc and BRISTOL-MYERS SQUIBB. Phases of this market are Phase II.
Key Questions Answered
- What are market estimates and forecasts; which of Bosutinib markets are doing well and which are not?
- What is the competitive landscape; How companies like Pfizer Inc, Novartis AG and ARIAD Pharmaceuticals Inc doing in Bosutinib?
- This reports provides most granular segmentation on Chromatography, E-Clinical Trial Solutions, Wireless Health and Clinical Trial Management Systems (CTMS).
- This report provides market sizing and forecast for the Bosutinib market. It also provides market sizing and forecast along with the drivers/inhibitors/opportunity analysis for each of the micro markets.
- The report provides deep dive competitive landscape covering the top players such as Pfizer Inc, Novartis AG, ARIAD Pharmaceuticals Inc and BRISTOL-MYERS SQUIBB.
- The reports provides benchmarking insight on the top players Pfizer Inc, Novartis AG, ARIAD Pharmaceuticals Inc and BRISTOL-MYERS SQUIBB.
- The report provide competitive intelligence on Pfizer Inc, Novartis AG, ARIAD Pharmaceuticals Inc and BRISTOL-MYERS SQUIBB.
- Global Bosutinib companies
- Manufacturing Companies
- Traders, distributors, and suppliers
- Governmental and research organizations
- Associations and industry bodies
- Technology providers
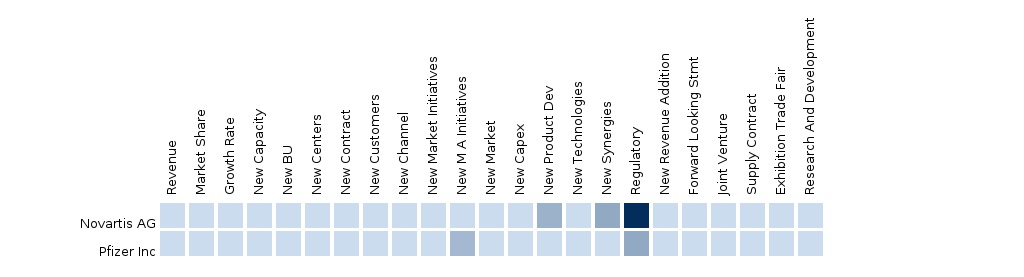
- European Medicines Agency accepted Pfizer's regulatory submissions.
- InVivoScribe Technologies Inc. and Novartis collaborated to develop and market a companion diagnostic test.
- Novartis obtained Canada healthcare authority approval for Tasigna(drug)
- Novartis received approval for Tasigna (treatment of chronic myelogenous leukemia) in Japan.
- Pfizer Inc. proposed regulatory submissions for a Marketing Authorization Application (MAA).
- Novartis obtained European Commission’s approval for Tasigna (nilotinib)
- Novartis received Japan's Ministry of Health, Labour and Welfare’s approval for Tasigna (nilotinib)
- Novartis reported data on phase III clinical trial study to demonstrate that Tasigna (nilotinib) shown better efficacy than Glivec (imatinib) in the treatment of adult Ph+ CML in chronic phase
- Cepheid collaborated with Novartis for the commercialization of a test to monitor Tasigna.
- Novartis received approval from The Swiss health authority for Tasigna (nilotinib)
What makes our report unique?
Audience for this report
Top developments
1 Introduction
1.1 Analyst Insights
1.2 Market Definitions
1.3 Market Segmentation & Aspects Covered
1.4 Research Methodology
2 Executive Summary
3 Market Overview
4 By Submarkets
4.1 Chromatography
4.2 E-Clinical Trial Solutions
4.3 Wireless Health
4.4 Clinical Trial Management Systems (CTMS)
4.5 Healthcare IT outsourcing
4.6 Immunoassay
4.7 Digital Pathology
4.8 Chromatography Instruments
4.9 Next Generation Sequencing (NGS)
4.10 Proteomics
5 By Technologies
5.1 Microfluidics
5.2 Chromatography Reagents
6 By Products
6.1 Lab-on-chips (Microfluidics)
6.2 Microreactors
6.3 AMG 103
6.4 DFP-10917
6.5 Tasigna
6.6 BP-100-1.01
7 By Phases
7.1 Phase II
8 By Geographies
8.1 USA
8.2 Canada
8.3 United Kingdom
8.4 Germany
8.5 France
8.6 Italy
8.7 Spain
8.8 Japan
9 By Companies
9.1 Pfizer Inc
9.2 Novartis AG
9.3 ARIAD Pharmaceuticals Inc
9.4 BRISTOL-MYERS SQUIBB
Please fill in the form below to receive a free copy of the Summary of this Report
Custom Market Research Services
We will customize the research for you, in case the report listed above does not
meet with your exact requirements. Our custom research will comprehensively cover
the business information you require to help you arrive at strategic and profitable
business decisions.
Please visit http://www.micromarketmonitor.com/custom-research-services.html to specify your custom Research Requirement
Please visit http://www.micromarketmonitor.com/custom-research-services.html to specify your custom Research Requirement














