You are here :
Home
Healthcare
Payment Link for Sterlitech Corporation - Track etched Membrane Market & Pharmaceutical Filtration Market
Drug formulations

Global Chronic lymphocytic leukemia (CLL) Market Research Report
Chronic lymphocytic leukemia (CLL) and its peer markets - T-cell Lymphoma, B-cell Lymphoma, Chronic myeloid leukemia (CML), Hairy-cell leukemia, Burkitt lymphoma, Lymphoplasmacytic lymphoma, Hodgkin lymphoma, Adult T-cell leukemia, Multiple myeloma and 3 other type markets - adds up to total Haematopoietic and lymphoid tissues Cancers market.
Chronic lymphocytic leukemia (CLL) can be segmented by Submarkets, Geographies, Companies and Products. Submarkets of this market are Chromatography, E-Clinical Trial Solutions, Wireless Health, Clinical Trial Management Systems (CTMS), Healthcare IT outsourcing, Immunoassay, Digital Pathology, Chromatography Instruments, Next Generation Sequencing (NGS), Proteomics, Marketed Drugs (CLL) and Pipeline Drugs (CLL). Geographies of this market are USA, Canada, United Kingdom, Germany, France, Italy, Spain and Japan. Companies of this market are Genzyme Corporation, Glaxosmithkline Plc, Celgene Corporation, Genmab A/S, Biogen Idec, F. Hoffmann-La Roche Ltd., Gilead Sciences, Tragara Pharmaceuticals, Inc., SBI Biotech., Boehringer Ingelheim GmbH, Genentech, Inc., MorphoSys, Novartis AG, AbbVie Inc., Ascenta Therapeutics, Inc., Merck & Co., Inc., Janssen Biotech, Inc., Pharmacyclics, Inc., CytRx, BioCryst Pharmaceuticals, Inc., Biothera, Memgen, LLC., Immunomedics,Inc., Cyclacel Pharmaceuticals, Inc., TG Therapeutics, Inc. , Emergent BioSolutions Inc. and Teva Pharmaceuticals, Inc (cephalon). Products of this market are DFP-10917.
Chronic lymphocytic leukemia (CLL) can be segmented by Submarkets, Geographies, Companies and Products. Submarkets of this market are Chromatography, E-Clinical Trial Solutions, Wireless Health, Clinical Trial Management Systems (CTMS), Healthcare IT outsourcing, Immunoassay, Digital Pathology, Chromatography Instruments, Next Generation Sequencing (NGS), Proteomics, Marketed Drugs (CLL) and Pipeline Drugs (CLL). Geographies of this market are USA, Canada, United Kingdom, Germany, France, Italy, Spain and Japan. Companies of this market are Genzyme Corporation, Glaxosmithkline Plc, Celgene Corporation, Genmab A/S, Biogen Idec, F. Hoffmann-La Roche Ltd., Gilead Sciences, Tragara Pharmaceuticals, Inc., SBI Biotech., Boehringer Ingelheim GmbH, Genentech, Inc., MorphoSys, Novartis AG, AbbVie Inc., Ascenta Therapeutics, Inc., Merck & Co., Inc., Janssen Biotech, Inc., Pharmacyclics, Inc., CytRx, BioCryst Pharmaceuticals, Inc., Biothera, Memgen, LLC., Immunomedics,Inc., Cyclacel Pharmaceuticals, Inc., TG Therapeutics, Inc. , Emergent BioSolutions Inc. and Teva Pharmaceuticals, Inc (cephalon). Products of this market are DFP-10917.
Key Questions Answered
- What are market estimates and forecasts; which of Chronic lymphocytic leukemia (CLL) markets are doing well and which are not?
- What is the competitive landscape; How companies like Genzyme Corporation, Glaxosmithkline Plc and Celgene Corporation doing in Chronic lymphocytic leukemia (CLL)?
- This reports provides most granular segmentation on Chromatography, E-Clinical Trial Solutions, Wireless Health and Clinical Trial Management Systems (CTMS).
- This report provides market sizing and forecast for the Chronic lymphocytic leukemia (CLL) market. It also provides market sizing and forecast along with the drivers/inhibitors/opportunity analysis for each of the micro markets.
- The report provides deep dive competitive landscape covering the top players such as Genzyme Corporation, Glaxosmithkline Plc, Celgene Corporation and Genmab A/S.
- The reports provides benchmarking insight on the top players Genzyme Corporation, Glaxosmithkline Plc, Celgene Corporation and Genmab A/S.
- The report provide competitive intelligence on Genzyme Corporation, Glaxosmithkline Plc, Celgene Corporation and Genmab A/S.
- Global Chronic lymphocytic leukemia (CLL) companies
- Manufacturing Companies
- Traders, distributors, and suppliers
- Governmental and research organizations
- Associations and industry bodies
- Technology providers
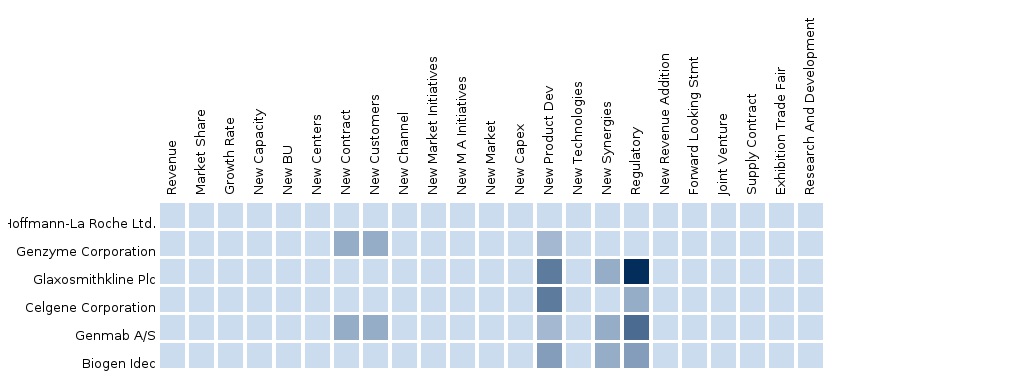
- Genmab collaborated with Seattle Genetics Inc. on a second antibody-drug conjugate (ADC) research
- Celgene reported clinical data from two investigational studies
- GSK and Genmab (Denmark) reported results of concluded pivotal trial of Arzerra (ofatumumab)
- Celgene Corporation obtained marketing authorization from Japan Ministry of Health, Labour and Welfare (MHLW) for Revlimid.
- Celgene Corporation presented data from investigational Phase II study of lenalidomide
- GSK and Genmab (Denmark) obtained European Commission (EC) grant for Arzerra
- GlaxoSmithKline and Genmab obtained European Commission (EC) grant for Arzerra
- Genentech Inc. and Biogen Idec received FDA approval for Rituxan (rituximab) in combination with fludarabine and cyclophosphamide (FC)
- GSK and Genmab (Denmark) from European Medicines Agency’s Committee positive opinion for Medicinal Products for Human Use (CHMP) Arzerra
- Genzyme declared data from CAM314 randomized phase III clinical trial of Campath (alemtuzumab) along with Fludara (fludarabine phosphate)
What makes our report unique?
Audience for this report
Top developments
1 Introduction
1.1 Analyst Insights
1.2 Market Definitions
1.3 Market Segmentation & Aspects Covered
1.4 Research Methodology
2 Executive Summary
3 Market Overview
4 By Submarkets
4.1 Chromatography
4.2 E-Clinical Trial Solutions
4.3 Wireless Health
4.4 Clinical Trial Management Systems (CTMS)
4.5 Healthcare IT outsourcing
4.6 Immunoassay
4.7 Digital Pathology
4.8 Chromatography Instruments
4.9 Next Generation Sequencing (NGS)
4.10 Proteomics
4.11 Marketed Drugs (CLL)
4.12 Pipeline Drugs (CLL)
5 By Products
5.1 DFP-10917
6 By Geographies
6.1 USA
6.2 Canada
6.3 United Kingdom
6.4 Germany
6.5 France
6.6 Italy
6.7 Spain
6.8 Japan
7 By Companies
7.1 Genzyme Corporation
7.2 Glaxosmithkline Plc
7.3 Celgene Corporation
7.4 Genmab A/S
7.5 Biogen Idec
7.6 F. Hoffmann-La Roche Ltd.
7.7 Gilead Sciences
7.8 Tragara Pharmaceuticals, Inc.
7.9 SBI Biotech.
7.10 Boehringer Ingelheim GmbH
7.11 Genentech, Inc.
7.12 MorphoSys
7.13 Novartis AG
7.14 AbbVie Inc.
7.15 Ascenta Therapeutics, Inc.
7.16 Merck & Co., Inc.
7.17 Janssen Biotech, Inc.
7.18 Pharmacyclics, Inc.
7.19 CytRx
7.20 BioCryst Pharmaceuticals, Inc.
7.21 Biothera
7.22 Memgen, LLC.
7.23 Immunomedics,Inc.
7.24 Cyclacel Pharmaceuticals, Inc.
7.25 TG Therapeutics, Inc.
7.26 Emergent BioSolutions Inc.
7.27 Teva Pharmaceuticals, Inc (cephalon)
Please fill in the form below to receive a free copy of the Summary of this Report
Custom Market Research Services
We will customize the research for you, in case the report listed above does not
meet with your exact requirements. Our custom research will comprehensively cover
the business information you require to help you arrive at strategic and profitable
business decisions.
Please visit http://www.micromarketmonitor.com/custom-research-services.html to specify your custom Research Requirement
Please visit http://www.micromarketmonitor.com/custom-research-services.html to specify your custom Research Requirement
| PRODUCT TITLE | PUBLISHED | |
|---|---|---|
 |
Campath/MabCampath Campath/MabCampath and Treanda, Arzerra, |
Upcoming |
 |
Treanda Treanda and Campath/MabCampath, Arzerra, |
Upcoming |
 |
Fludara Fludara and Campath/MabCampath, Treanda, |
Upcoming |
 |
FCR Regimen FCR Regimen and Campath/MabCampath, Treanda, |
Upcoming |
 |
FC Regimen FC Regimen and Campath/MabCampath, Treanda, |
Upcoming |
 |
Phase I (CLL) Phase I (CLL) and Phase II (CLL) and Phase III (CLL) adds up to total... |
Upcoming |
 |
Phase II (CLL) Phase II (CLL) and Phase I (CLL) and Phase III (CLL) adds up to total... |
Upcoming |
 |
Phase III (CLL) Phase III (CLL) and Phase I (CLL) and Phase II (CLL) adds up to total... |
Upcoming |
8 reports |
Show













